3D model is for representation purpose only.
.
.
 |
 |
 |
 |
 |
|---|---|---|---|---|
| TKPR | 6-aminohexanoic acid | 3-[(1-Nitroacridin-9-yl)amino]propanoic acid (nitroacrin) derivative |
| Identification | |
|---|---|
| ConjuPepDB ID | cpd00822 |
| Smiles | |
| Inchi Key | InChIKey=CNZVZEMDDDBBQW-DIXVVABASA-N |
| Molecular weight | 962.534 |
| Molecular formula | C47H70N12O10 |
| Chemical name | Peptide information |
| Sequence (one letter) | TKPR |
| Length | 4 |
| Peptide name | tuftsin (liberated from the Fc domain of the heavy chain of IgG |
| External ID | |
|---|---|
| CAS number | 1283096-56-9 |
| Other information | |
|---|---|
| Application | Anticancer therapy, A549 and HL-60 cells) |
| Additional function | Increase activity and specific toxicity |
| Conjugate type | amide |
| Linker | N/A |
| Linker Type | 6-aminohexanoic acid |
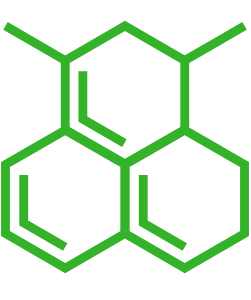
| Small molecule | 3-[(1-Nitroacridin-9-yl)amino]propanoic acid (nitroacrin) derivative |
| Small molecule CAS | 81483-74-1 |
| Small molecule structure | |
| Structure method | 1H-NMR |
| Calculated properties | |
|---|---|
| LogP | -0.16462 |
| Rotatable bonds | 28 |
| H bond donor | 10 |
| H bond acceptor | 16 |
| Polar surface area (PSA) | 355.40000 |
| Citations | |||||
|---|---|---|---|---|---|
| ID | Title | Year | Authors | Journal | DOI |
Solid phase synthesis and biological activity of tuftsin conjugates | 2011 | Kukowska-Kaszuba, Magdalena; Dzierzbicka, Krystyna; Serocki, Marcin; Skladanowski, Andrzej | Journal of Medicinal Chemistry | ||