3D model is for representation purpose only.
.
.
 |
 |
 |
 |
 |
|---|---|---|---|---|
| LARKFEAFARAG | Coumarine, 7-Methoxy-2-oxo-2H-1-benzopyran-3-carboxylic acid |
| Identification | |
|---|---|
| ConjuPepDB ID | cpd00616 |
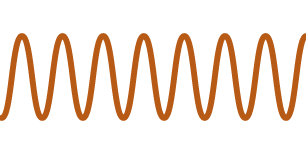
| Smiles | |
| Inchi Key | InChIKey=DMQZKZXWUUWSPF-AZMIHLOHSA-N |
| Molecular weight | 1536.78 |
| Molecular formula | C72H104N20O18 |
| Chemical name | Peptide information |
| Sequence (one letter) | LARKFEAFARAG |
| Length | 12 |
| Peptide name | dodecapeptide |
| External ID | |
|---|---|
| CAS number | 1370726-92-3 |
| Other information | |
|---|---|
| Application | Synthesis |
| Additional function | Assembling functional peptide bioconjugates |
| Conjugate type | amide |
| Linker | no |
| Small molecule | Coumarine, 7-Methoxy-2-oxo-2H-1-benzopyran-3-carboxylic acid |
| Small molecule CAS | 20300-59-8 |
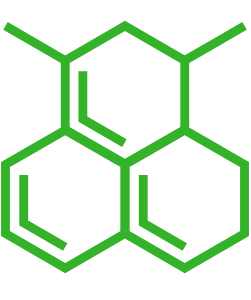
| Small molecule structure | |
| Structure method | 1H-, 13C- and 19F-NMR |
| Calculated properties | |
|---|---|
| LogP | -6.56674 |
| Rotatable bonds | 47 |
| H bond donor | 19 |
| H bond acceptor | 24 |
| Polar surface area (PSA) | 619.94000 |
| Citations | |||||
|---|---|---|---|---|---|
| ID | Title | Year | Authors | Journal | DOI |
Efficient formation of heterodimers from peptides and proteins using unsymmetrical polyfluorophenyl esters of dicarboxylic acids | 2012 | Slosarczyk, Adam T.; Baltzer, Lars | Journal of Peptide Science | ||