3D model is for representation purpose only.
.
.
 |
 |
 |
 |
 |
|---|---|---|---|---|
| GFLG | doxorubicine |
| Identification | |
|---|---|
| ConjuPepDB ID | cpd00261 |
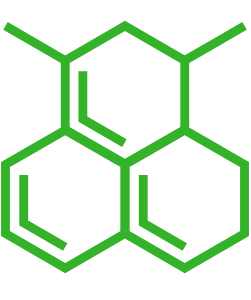
| Smiles | |
| Inchi Key | InChIKey=PDFQPTQAJVLHNY-AOOUAPNOSA-N |
| Molecular weight | 985.396 |
| Molecular formula | C50H59N5O16 |
| Chemical name | Peptide information |
| Sequence (one letter) | GFLG |
| Length | 4 |
| Peptide name | tetrapeptide |
| External ID | |
|---|---|
| CAS number | 161261-02-5 |
| Other information | |
|---|---|
| Application | Anticancer therapy, prostate (DU145 cells) |
| Additional function | MMP-2-cleavable linker, enhance tumor-targeting and -penetrating ability |
| Pharmacological class | anticancer |
| Conjugate type | amide |
| Linker | no |
| Small molecule | doxorubicine |
| Small molecule CAS | 23214-92-8 |
| Small molecule structure | |
| Calculated properties | |
|---|---|
| LogP | 2.10170 |
| Rotatable bonds | 19 |
| H bond donor | 10 |
| H bond acceptor | 16 |
| Polar surface area (PSA) | 325.55000 |
| Citations | |||||
|---|---|---|---|---|---|
| ID | Title | Year | Authors | Journal | DOI |
Enhancing Accumulation and Penetration of HPMA Copolymer-Doxorubicin Conjugates in 2D and 3D Prostate Cancer Cells via iRGD Conjugation with an MMP-2 Cleavable Spacer | 2015 | Peng, Zheng-Hong; Kopecek, Jindrich | Journal of the American Chemical Society | ||