3D model is for representation purpose only.
.
.
 |
 |
 |
 |
 |
|---|---|---|---|---|
| SRSELIVHQRLF | biotin |
| Identification | |
|---|---|
| ConjuPepDB ID | cpd00251 |
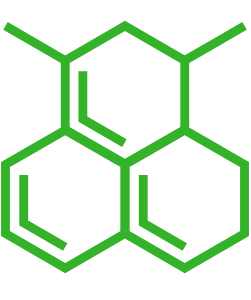
| Smiles | |
| Inchi Key | InChIKey=UZVQDXZSAFDENK-CGMXJCLRSA-N |
| Molecular weight | 1934.13 |
| Molecular formula | C90H151N25O20S |
| Chemical name | Peptide information |
| Sequence (one letter) | SRSELIVHQRLF |
| Length | 12 |
| Peptide name | antimicrobial peptide Cm-p5 |
| External ID | |
|---|---|
| CAS number | 1781222-39-6 |
| Other information | |
|---|---|
| Application | Synthesis, incorporation of complex N-substituents at both termini and at internal positions |
| Additional function | Modifications of biologically relevant peptides: fluorescent labeling, PEGylation, glycosylation, etc. |
| Pharmacological class | other |
| Conjugate type | amide |
| Linker | no |
| Small molecule | biotin |
| Small molecule CAS | 58-85-5 |
| Small molecule structure | |
| Calculated properties | |
|---|---|
| LogP | -4.93676 |
| Rotatable bonds | 69 |
| H bond donor | 24 |
| H bond acceptor | 27 |
| Polar surface area (PSA) | 732.06000 |
| Citations | |||||
|---|---|---|---|---|---|
| ID | Title | Year | Authors | Journal | DOI |
Aminocatalysis-Mediated on-Resin Ugi Reactions: Application in the Solid-Phase Synthesis of N-Substituted and Tetrazolo Lipopeptides and Peptidosteroids | 2015 | Morales, Fidel E.; Garay, Hilda E.; Munoz, Daniela F.; Augusto, Yarelys E.; Otero-Gonzalez, Anselmo J.; Reyes Acosta, Osvaldo; Rivera, Daniel G. | Organic Letters | ||