3D model is for representation purpose only.
.
.
 |
 |
 |
 |
 |
|---|---|---|---|---|
| TKPKGTKPKGTKPKGTKPKG | glycolic acid | N-(4-Acetylphenyl)-5-bromo-2-hydroxybenzamide |
| Identification | |
|---|---|
| ConjuPepDB ID | cpd00129 |
| Smiles | |
| Inchi Key | InChIKey=MMYZOAGZZUYPKI-NHFOEDLHSA-N |
| Molecular weight | 2450.28 |
| Molecular formula | C109H180BrN31O28 |
| Chemical name | Peptide information |
| Sequence (one letter) | TKPKGTKPKGTKPKGTKPKG |
| Length | 20 |
| Peptide name | N/A |
| External ID | |
|---|---|
| CAS number | 2100270-30-0 |
| Other information | |
|---|---|
| Application | Antimicrobial therapy, anti-tuberculosis |
| Additional function | Target cell-directed delivery and fluorescent labeling |
| Pharmacological class | antimicrobial, antibacterial |
| Conjugate type | amide |
| Linker | N/A |
| Linker Type | glycolic acid |
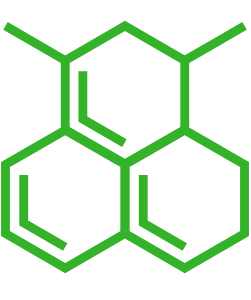
| Small molecule | N-(4-Acetylphenyl)-5-bromo-2-hydroxybenzamide |
| Small molecule CAS | 1007653-77-1 |
| Small molecule structure | |
| Structure method | 1H- and 13C-NMR |
| Calculated properties | |
|---|---|
| LogP | -13.34487 |
| Rotatable bonds | 78 |
| H bond donor | 31 |
| H bond acceptor | 37 |
| Polar surface area (PSA) | 949.93000 |
| Citations | |||||
|---|---|---|---|---|---|
| ID | Title | Year | Authors | Journal | DOI |
In vitro biological evaluation of new antimycobacterial salicylanilide-tuftsin conjugates | 2017 | European Journal of Medicinal Chemistry | European Journal of Medicinal Chemistry | ||